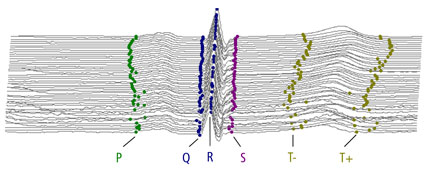
Regulatory Recommendations
Recent regulatory recommendations (ICH-E14) have significantly
impacted the way that studies are designed. For the purpose of a
„thorough QT study“ thousands of ECGs have to be recorded in a short
period of time. Modern technical equipment enables us to deal with
huge volumes of ECG data. Our capacity to evaluate ECGs currently
exceed 20.000 recordings per month.
Phase I-IV clinical trials
ExCard Research has specialized in the analysis and interpretation
of ECGs from phase I-IV clinical trials using:
| > |
12-lead ECGs
(including continuous, high-resolution 12- lead Holter recordings)
for Phase I–IV studies and thorough QT trials |
| > |
arrhythmia
analysis from 24- or 48-hour Holter recordings |
|
Evaluating new parameters
As ExCard Research is a scientifically driven ECG core lab, we
are
continuously looking for new ECG parameters that may help to assess
the cardiac safety of new drugs.
New parameters on which we currently focus include:
| > |
beat-to-beat
variability of the QT interval (including Poincare plotting)
|
| > |
beat-to-beat-assessment
of the area under the T wave |
| > |
assessment
of the restitution and hysteresis of the QT interval |
| > |
categorization
of T wave morphology and assessment of the dynamics
of beat-to-beat changes T wave morphology |
|
Advanced analysis options
We also offer the following analyses:
| > |
heart rate
variability (HRV) in time- and frequency-domain |
| > |
evaluation
of atrial fibrillation cycle length (AFCL) |
| > |
Implantable
cardioverter defibrillator (ICD) memory analysis. Visualization
and analysis of stored electrograms and documentation of the
rate and detection criteria |
These analyses are performed using the following ECG recording
options:
| > |
standard 12-lead
recorders |
| > |
24-hours,
12-lead digital continuous recorders |
| > |
48-hours, 12-lead
digital continuous recorders |
| > |
24-hours, 12-lead
digital continuous high-resolution recorders
(1024 Hz) |
|